The National Agency for Food and Drug Administration and Control (NAFDAC) has approved the emergency use of Oxford/AstraZeneca COVID-19 vaccine in the country.
The vaccine was recently approved by the W.H.O for emergency use.
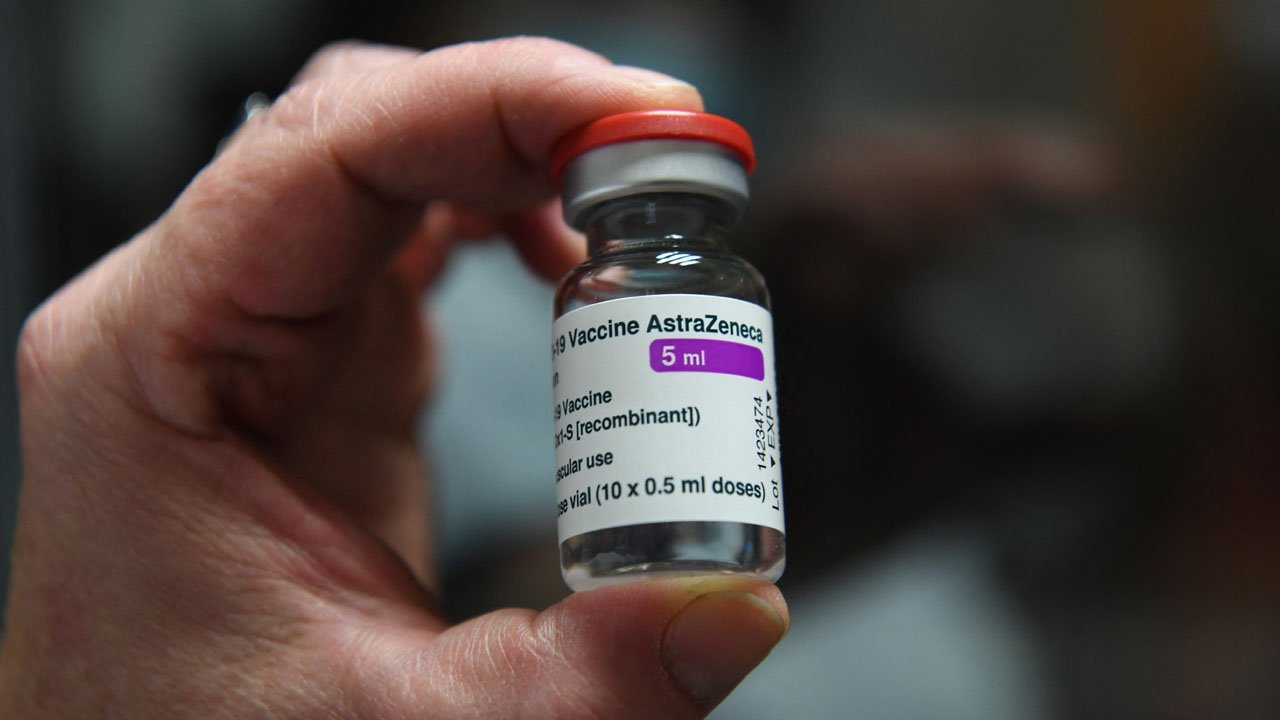
Director-General of NAFDAC, Dr. Mojisola Adeyeye who announced this yesterday in Abuja NAFDAC observed that the agency got the dossier of the vaccine a week ago adding that the NAFDAC safety committee went to work immediately to evaluate its safety and efficacy for Nigerians.
She explained that the Oxford/AstraZeneca vaccine, also known as ChAdOx1 nCoV-19, or AZD1222, is a viral vector vaccine. Scientists used an adenovirus, originally derived from chimpanzees, and modified it with the aim of training the immune system to mount a strong response against SARS-CoV-2 (the virus that causes COVID-19).
Adeyeye noted that the recommendation for Emergency Use Authorization was based on rigorous scientific considerations such as vaccine quality, vaccine safety and efficacy, dose pack and storage.
She stated that the active substance for the vaccine is manufactured and controlled by the Serum Institute of India Private Limited (SIIPL). A GMP certificate and manufacturing license issued by the India National Regulatory Authority (NRA) has been presented and found to be authentic and valid.
The NAFDAC boss said that the multidose (2 doses or 10 doses) vial is stored at 2-8OC, one dose (0.5ml) contains 5 x 1010 virus particle. On available stability data, the applicant has proposed a drug product shelf life of 6 months.
On the vaccine safety and efficacy, Adeyeye stated that from the Phases 2/3 conducted, COVISHIELD was found safe and well-tolerated in adults above 18 years of age and the incidence of solicited, unsolicited AEs and SAEs were comparable in the study control. groups. No causally related SAE was caused by the study vaccine.
According to her, the guidance addressed many regulatory issues including the submission of dossiers by manufacturers and Market authorization holder, assessment of Dossier through different mechanisms and using the common technical document for the rolling submission, emergency Use Authorization expedited approval, full review
Post-marketing Pharmacovigilance and Surveillance.
She further noted that the Agency will be using its recently launched Med Safety App for Active Pharmacovigilance of the vaccines in collaboration with the sister agencies stressing that NAFDAC plans to also use the Traceability with GS1 technology to monitor the vaccine distribution using Global Trade Item Number (GTIN) to prevent fake vaccines from infiltrating the supply chain and to ensure there is no diversion. This effort will create a reliable and predictable supply chain.
Adeyeye said that tt the continental level, NAFDAC is on the Regulators Steering Committee of African Union -3S (Smart, Safety Surveillance) with Ghana, South Africa and Ethiopia. The committee was formed as a preparatory caucus for safety monitoring of medicines but using COVID-19 vaccine as a pilot.
“Pharmacovigilance of Covid-19 Vaccines Safety of the vaccine is premium to NAFDAC and a lot of efforts are being put into this regulatory function. The Agency initiated a multi-stakeholder collaboration with National Primary Health Care Development Agency (NPHCDA), Nigeria Center for Disease Control (NCDC), UNICEF, GAVI, WHO and Ministry of Health. The focus is to use a holistic approach for the effective immunization or delivery of the vaccines and monitor any Adverse Events Following Immunisation (AEFI). The multi-stakeholder technical working groups have been meeting to address different issues, from access to distribution to traceability (track and trace) of the vaccines to monitoring of adverse events following immunization”.
“The use of AstraZeneca/Oxford COVID-19 vaccine, a recombinant ChAdOx1 adenoviral vector encoding the structural surface glycoprotein (Spike protein) antigen of the SARS-CoV-2 was given an approval for Emergency Use Listing by WHO on Monday, February 15, 2021. WHO’s Emergency Use Listing (EUL) assesses the quality, safety and efficacy of COVID-19 vaccines and is a prerequisite for COVAX Facility vaccine supply. It also allows countries to expedite their own regulatory approval to import and administer COVID-19 vaccines. The EUL will allow Nigeria to receive the first batch of the vaccine from COVAX Facility within weeks subject to approval by NAFDAC”, she added.
According to her, COVAX facility which is the vaccines pillar of the Access to COVID-19 Tools (ACT) Acceleratoris jointly led by The Global Alliance for Vaccines and Immunisation (GAVI), the Coalition for Epidemic Preparedness Innovations (CEPI) and the World Health Organization (WHO). ‘Its aim is to accelerate the development and manufacture of COVID-19 vaccines and to guarantee fair and equitable access for every country in the world. COVAX is also poised to ensure that ninety- two (92) eligible Low- and Middle-Income Countries ( LMIC) get access to COVID-19 vaccines through GAVI. Nigeria is one of the eligible countries.
Adeyeye observed that NAFDAC has been preparing for the COVID-19 vaccines and vaccination since the early phase of the pandemic – around April 2020 and was positioned to a great extent to work online during the lockdown due to digitalization of many of our processes and continuous improvement.
Global meetings have been held continuously since April with a focus on understanding the disease and the advent of vaccines. The Director-General has been meeting every other week with International Coalition of Medicine Regulatory Authorities (ICMRA) while DG and respective Directors and staff have been meeting with WHO, African Medicine Regulatory Harmonisation (AMRH), WA-MRH, WAHO, AVAREF, etc.
The Agency established the COVID-19 Vaccine Committee and has been busy developing guidelines and guidance. NAFDAC is the first National Regulatory Agency in Africa to have Guidance on Regulatory Preparedness for EUA, Licensing or Access to COVID-19 Vaccines
https://www.nafdac.gov.ng/wp-content/uploads/Files/Resources/Guidelines/DRUG_GUIDELINES/Guidance-document-on-Covid-19-vaccine-preparedness-finalized.pdf
guardian.ng






